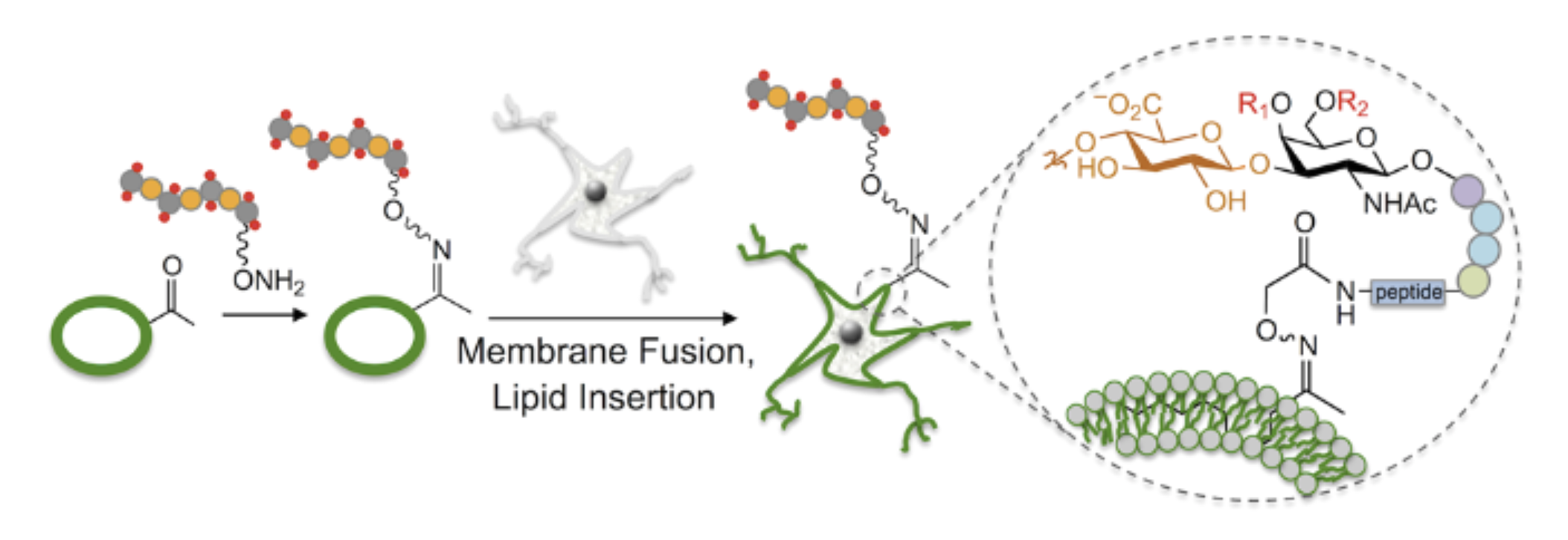
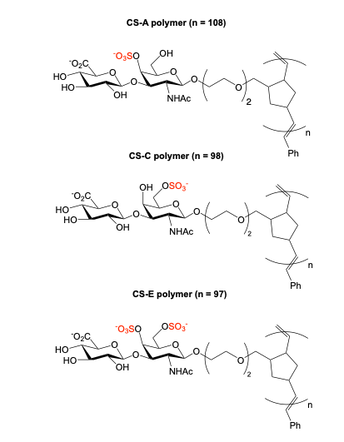
Neuroplasticity and chondroitin sulfate
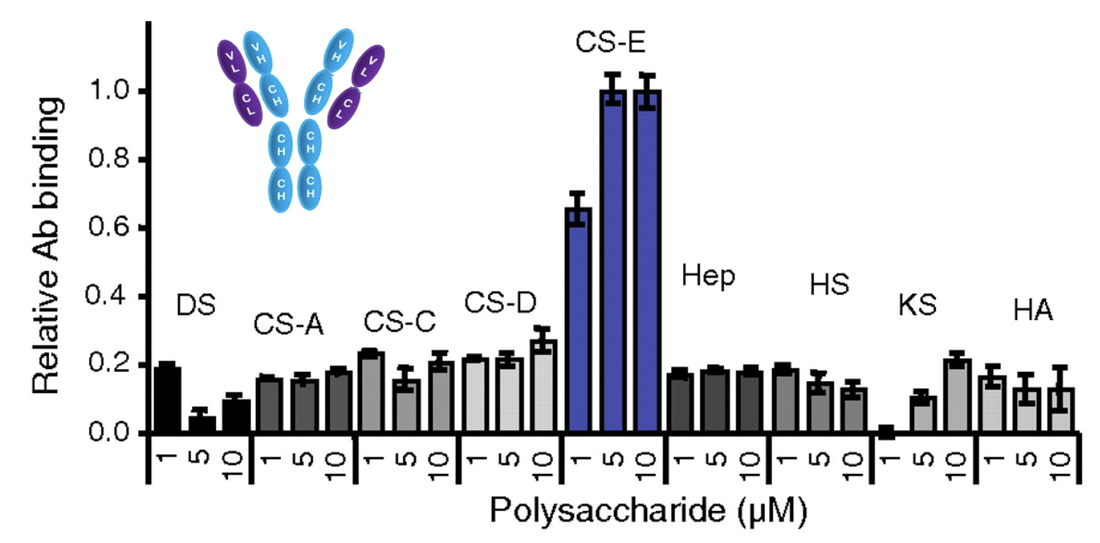
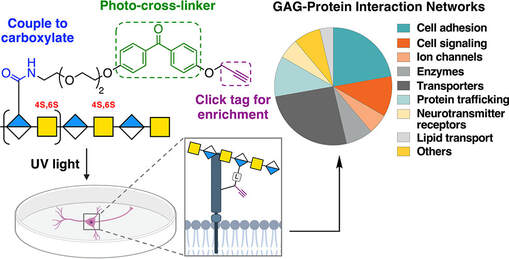
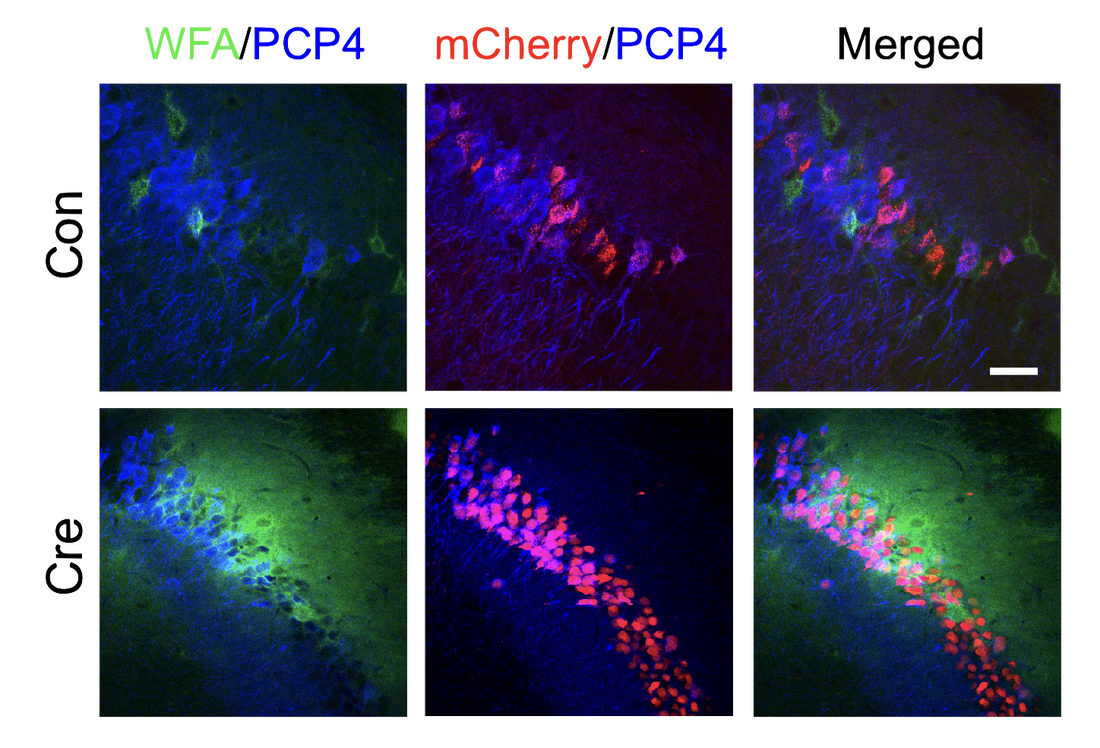
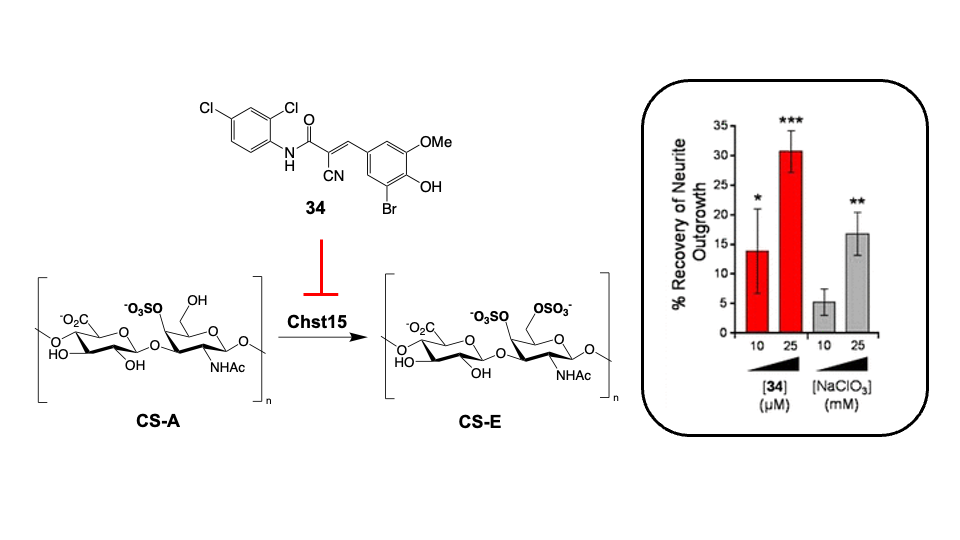
Chondroitin sulfate glycosaminoglycans (CS GAGs) are a family of linear, sulfated polysaccharides that are covalently linked to core proteins to form CS proteoglycans (CSPGs) on the cell surface. In the central nervous system (CNS), CSPGs make up a large portion of the extracellular matrix and interact with hundreds of structural proteins and signaling molecules to regulate key processes such as cellular maturation and differentiation, neuroinflammation, and neuronal growth during development. As part of our characterization of the structure-function relationships of CS, our lab identified the CS sulfation motif E (CS-E) inhibits neuronal regrowth and recovery. This motif is found abundantly in glial scars that form after CNS injury, and ablation of the CS-E motif results in a restored ability for neurons to regrow axons. These findings highlight the therapeutic potential of studying CS GAGs as potential targets for intervention. Thus, our lab is focused on continuing to develop new tools to facilitate discovery of other functions of CS GAGs and highlight avenues for stimulating neuronal recovery in CNS injury and neurodegenerative disease.